Manufacturer Partnership Program
Our Valued Partners

Title
NSF Certification

Title
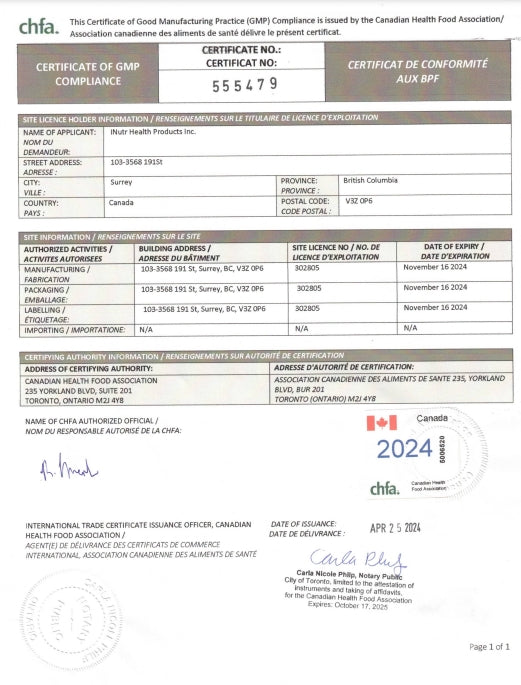
GMP Certification
Title
FDA Certification

Title
Title
USDA Organic Certification

Title
Your Benefits
Low-Risk Market Entry
Reduced MOQs
Global Distribution
Data Insights
Title
Manufacturer Requirements
Title
